Spatially Resolved Chemistry through Vibrational Microscopy
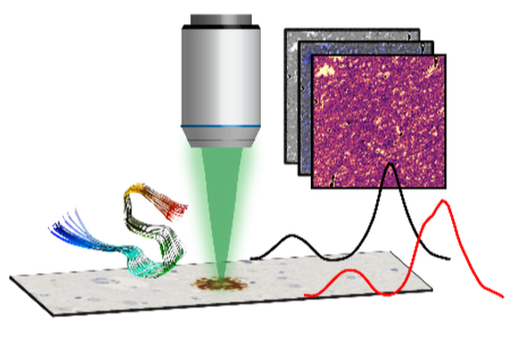
Our approach relies on vibrational spectroscopies augmented with microscopy modalities that enable mapping chemical and structural signatures of individual members of an ensemble and thus go beyond acquiring and interpreting average spectral signatures of complex heterogeneous systems.
For investigation of individual nanoscale amyloid aggregates, MOF crystals and self-assembled monolayers, we employ photothermal AFM-IR, which integrates IR spectroscopy with Atomic Force Microscopy (AFM) and circumvents diffraction limits to spatial resolution by using the AFM cantilever to detect photothermal expansion arising from resonant IR excitation the sample. AFM-IR thus offers the best of both worlds: spatial resolution of AFM and spectral sensitivity of IR and is an ideal technique for resolving the heterogeneity of amyloid aggregates and their evolution over the course of aggregation in-vitro.
Of course, for ex-vivo tissue specimens and polycrystalline films, AFM based approaches are not suitable, and to that end, we employ confocal IR spectroscopic imaging, which combines a confocal scanning microscope with an infrared laser source for illumination. We complement confocal IR imaging with optical photothermal IR microscopy (O-PTIR), which is based on the same principles of photothermal AFM-IR but uses the change in refractive index of a visible probe beam in an optical microscope to detect the IR signal and offers a ten-fold increase in resolution.
This approach enables seamless continuous integration of modalities from nanometers to millimeters as necessary while maintaining the same spectroscopic contrast and allows us to characterize individual fibrils to and amyloid plaques in brain tissues.
To complement the insights from IR, we also use Raman microspectroscopy, which integrates a Raman spectrometer with optical microscopy and allows us to gain comprehensive chemical insights into samples of interest.
For investigation of individual nanoscale amyloid aggregates, MOF crystals and self-assembled monolayers, we employ photothermal AFM-IR, which integrates IR spectroscopy with Atomic Force Microscopy (AFM) and circumvents diffraction limits to spatial resolution by using the AFM cantilever to detect photothermal expansion arising from resonant IR excitation the sample. AFM-IR thus offers the best of both worlds: spatial resolution of AFM and spectral sensitivity of IR and is an ideal technique for resolving the heterogeneity of amyloid aggregates and their evolution over the course of aggregation in-vitro.
Of course, for ex-vivo tissue specimens and polycrystalline films, AFM based approaches are not suitable, and to that end, we employ confocal IR spectroscopic imaging, which combines a confocal scanning microscope with an infrared laser source for illumination. We complement confocal IR imaging with optical photothermal IR microscopy (O-PTIR), which is based on the same principles of photothermal AFM-IR but uses the change in refractive index of a visible probe beam in an optical microscope to detect the IR signal and offers a ten-fold increase in resolution.
This approach enables seamless continuous integration of modalities from nanometers to millimeters as necessary while maintaining the same spectroscopic contrast and allows us to characterize individual fibrils to and amyloid plaques in brain tissues.
To complement the insights from IR, we also use Raman microspectroscopy, which integrates a Raman spectrometer with optical microscopy and allows us to gain comprehensive chemical insights into samples of interest.