Investigating Amyloid Plaques in Alzheimer's Disease Using IR and Raman Microscopy
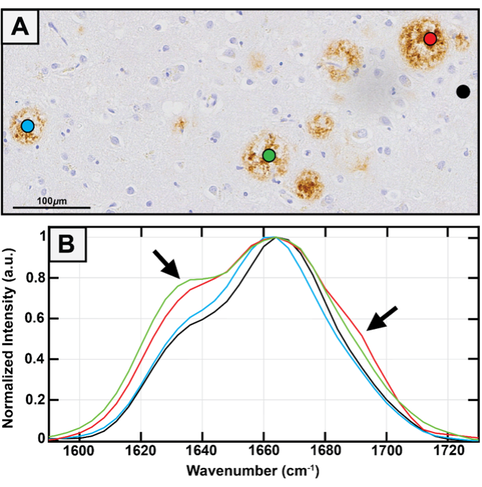
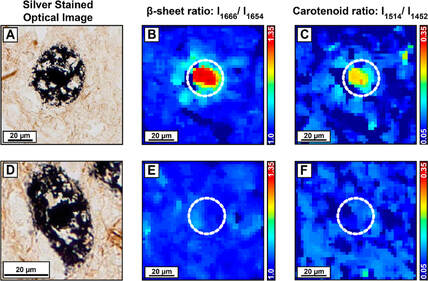
Although amyloid plaques are a well-known pathological marker of Alzheimer's Disease (AD), detailed structural information about Aβ assemblies in the human brain is lacking, and it is unclear to what extent different conformations identified in vitro persist in Aβ deposits in brain tissues and how their abundance correlates with AD. This is equally applicable to neurofibrillary tangles, which are aggregates of the tau protein, and another key pathological hallmark of AD and other neurodegenerative diseases. This gap in knowledge largely stems from the inability to extend conventional methods in structural biology towards spatially resolved imaging in tissues. In absence of spatial resolution, it is impossible to identify spectral signatures of specific plaques, and any differences therein. IR spectroscopy is one of the most widely used spectroscopic techniques for investigating amyloid structure, and it is thus natural to harness its capabilities towards characterizing the chemistry of plaques.
We use photothermal and confocal IR imaging along with Raman microspectroscopy to characterize the distribution of Aβ and tau secondary structures in AD brain tissue sections and compare these insights to structures seen in in-vitro aggregates to build a bridge between tin-vitro models and amyloid structures that exists in the human brain. Our goal is to categorize amyloid plaques based on their chemistry and secondary structure, and identify the true neurotoxic class of aggregates that are correlated to disease progression, which is of pivotal importance for design of therapeutics for AD.
We use photothermal and confocal IR imaging along with Raman microspectroscopy to characterize the distribution of Aβ and tau secondary structures in AD brain tissue sections and compare these insights to structures seen in in-vitro aggregates to build a bridge between tin-vitro models and amyloid structures that exists in the human brain. Our goal is to categorize amyloid plaques based on their chemistry and secondary structure, and identify the true neurotoxic class of aggregates that are correlated to disease progression, which is of pivotal importance for design of therapeutics for AD.