Nanoscale Infrared Spectroscopy of Fibrils by AFM-IR
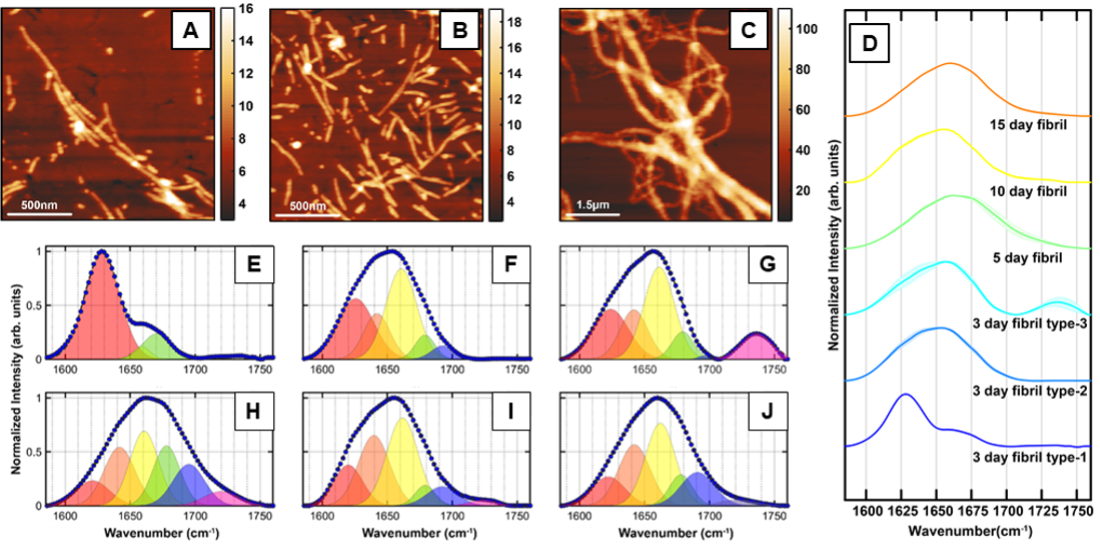
The misfolding and aggregation of tau proteins into fibrillar aggregates is the pathological hallmark of many neuro- degenerative diseases called tauopathies, including Alzheimer’s and Parkinson’s disease. Tau is a microtubule (MT)- associated protein that misfolds into insoluble cellular deposits called neurofibrillary tangles (NFTs). It is known that tau filaments in NFTs have a cross-β structure similar to that of amyloid fibrils. In the human brain, six different tau isoforms have been identified that differ with respect to the number of amino acid residues. The dominant isoform and fibrillar structure can vary with disease. All the tau isoforms are large polypeptides and, therefore, exhibit structural flexibility. Furthermore, tau and its isoforms exhibit polymorphism: essentially the same peptide aggregates into different fibrillar structures that differ not only with respect to morphology but also with respect to molecular arrangement. All of the above factors make isolation and structural analysis of specific tau aggregates formed at different stages of the aggregation a difficult task. In particular, the structural aspects of early-stage tau intermediates are not well-understood.
The aggregation of the amyloid beta (Aβ) protein into insoluble deposits or plaques is one of the main pathological features of Alzheimer’s disease (AD). However, the exact role of Aβ plaques in AD pathogenesis and the onset of dementia is unclear. The aggregation of different Aβ isoforms in vitro has been studied at length both experimentally and computationally, and similar to tau, while the structure of fibrils is well known, but the aggregation pathway and the intermediates that lead to fibrillar aggregates, particularly at early stages of aggregation, are not fully understood. Isolation and structural analysis of the amyloid aggregates formed at different stages of the aggregation still remain a challenge. The variations in protein structure within one subgroup of aggregates corresponding to a specific time point of aggregation are yet to be well understood. This is particularly relevant for early-stage aggregates such as oligomers and protofibrils and little is known about structural polymorphism of these early-stage aggregates. Essentially, it is not understood if a particular type of aggregate can exhibit different secondary structures and if/how such structural heterogeneity can influence later-stage aggregates.
Without the ability to spatially resolve individual aggregates, it is difficult to unequivocally attribute spectral features to specific species or morphologies or determine which transient species evolve into a particular morphology. Essentially it is not always possible to determine if observed spectral features arise from a particular aggregation state or a statistical mixture of different conformations. We address these challenges, we leverage the capabilities of AFM-IR and investigate individual fibrils and oligomers to uncover their unique structural facets and identify the transitions underlying the evolution of these species in the course of amyloid aggregation.
The aggregation of the amyloid beta (Aβ) protein into insoluble deposits or plaques is one of the main pathological features of Alzheimer’s disease (AD). However, the exact role of Aβ plaques in AD pathogenesis and the onset of dementia is unclear. The aggregation of different Aβ isoforms in vitro has been studied at length both experimentally and computationally, and similar to tau, while the structure of fibrils is well known, but the aggregation pathway and the intermediates that lead to fibrillar aggregates, particularly at early stages of aggregation, are not fully understood. Isolation and structural analysis of the amyloid aggregates formed at different stages of the aggregation still remain a challenge. The variations in protein structure within one subgroup of aggregates corresponding to a specific time point of aggregation are yet to be well understood. This is particularly relevant for early-stage aggregates such as oligomers and protofibrils and little is known about structural polymorphism of these early-stage aggregates. Essentially, it is not understood if a particular type of aggregate can exhibit different secondary structures and if/how such structural heterogeneity can influence later-stage aggregates.
Without the ability to spatially resolve individual aggregates, it is difficult to unequivocally attribute spectral features to specific species or morphologies or determine which transient species evolve into a particular morphology. Essentially it is not always possible to determine if observed spectral features arise from a particular aggregation state or a statistical mixture of different conformations. We address these challenges, we leverage the capabilities of AFM-IR and investigate individual fibrils and oligomers to uncover their unique structural facets and identify the transitions underlying the evolution of these species in the course of amyloid aggregation.
Related Publications
- Banerjee, S.; Baghel, D.; Pacheco de Oliveira, A.; Ghosh, A. β-Carotene, a Potent Amyloid Aggregation Inhibitor, Promotes Disordered Aβ Fibrillar Structure. International Journal of Molecular Sciences, 2023, 24(6), 5175. https://doi.org/10.3390/ijms24065175
- Banerjee, S.; Naik, T.; Baghel, D.; Ghosh, A. Intermediate Antiparallel Fibrils in Aβ40 Dutch Mutant Aggregation: Insights from Nanoscale Infrared Spectroscopy. Journal of Physical Chemistry B, 2023, 127(26), 5799. https://doi.org/10.1021/acs.jpcb.3c01869
- Banjeree, S.; Baghel, D.; Hasan Ul Iqbal, M.; Ghosh, A. Nanoscale Infrared Spectroscopy Identifies Parallel to Antiparallel β-Sheet Transformation of Aβ Fibrils. Journal of Physical Chemistry Letters, 2022, 13(45), 10522. doi.org/10.1021/acs.jpclett.2c02998
- Banjeree, S.; Ghosh, A. Structurally Distinct Polymorphs of Tau Aggregates Revealed by Nanoscale Infrared Spectroscopy. Journal of Physical Chemistry Letters, 2021, 12(45), 11035. https://doi.org/10.1021/acs.jpclett.1c02660